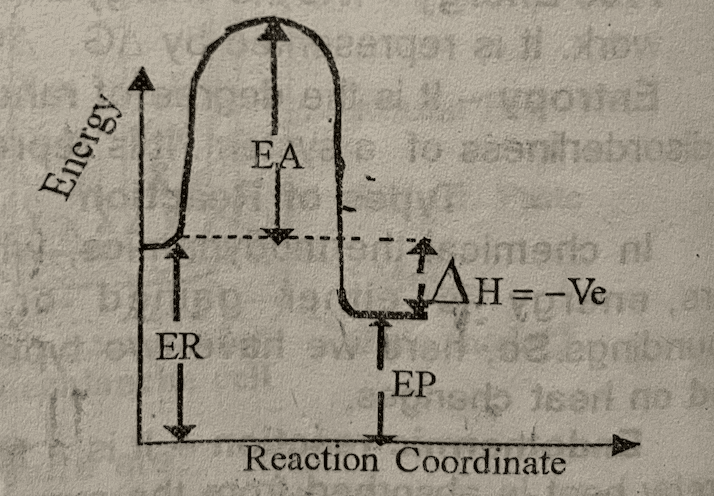
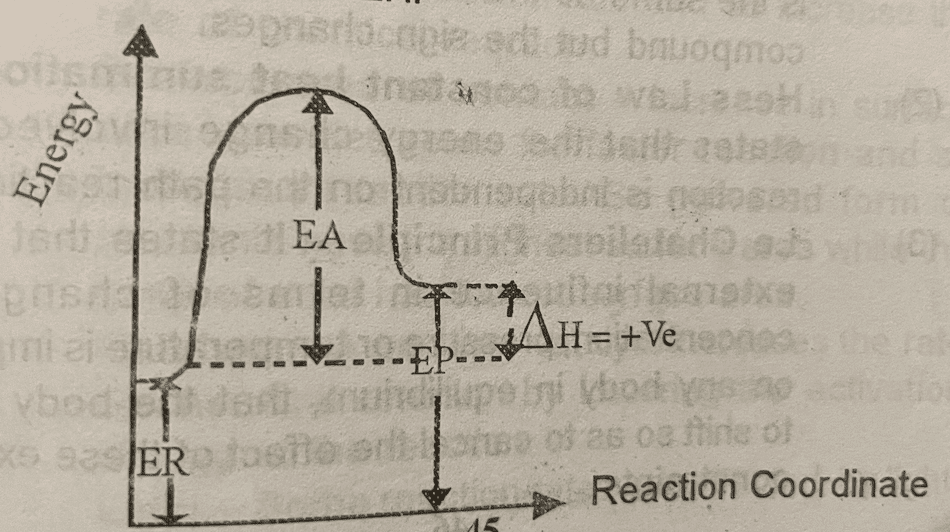
In chemical thermodynamics, when a reaction occurs, energy is either gained or lost to the surroundings. So, here we have two types of reactions based on heat changes.
(1) Endothermic reaction – It is a form of reaction whereby heat is absorbed from the surroundings by the system. Here, the enthalpy change is positive. So, the energy of the reactant is less than that of the product.
ΔH = Energy of product – Energy of reactant.
ΔH = ΔHp – ΔHr
Where: EA = Activation Energy
ER = Energy of reactant
EP = Energy of product
ΔH = Enthalpy change
(2) Exothermic reaction – It is a form of reaction whereby heat is liberated to surroundings by the system, So, the energy of the reactant is greater than the energy of the product. Hence, the enthalpy change is negative.
ΔH = Energy of product – Energy of reactant
ΔH = ΔHP – ΔHr
ΔH = Ve