Since each of the constituents of a mixture still retains its individual property, we can utilize this to separate them.
Sieving
It is a separation technique that is used to separate solids of different sizes. It is employed in garri processing industries and other industries where sieving is highly needed.
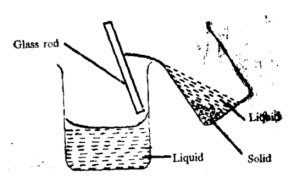
Decantation
It is used in separating a mixture containing liquid and solid particles into two distinct layers on standing.
Magnetic separation
It is used to separate magnetic substances from non-magnetic ones. it is employed in steel industries.
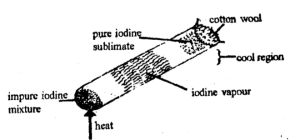
Sublimation
It Is the process whereby a substance changes directly from solid to gaseous state without passing through the liquid state. So, it is employed in the separation of any substance that sublime like iodine and. ammonium chloride (NH4CL).
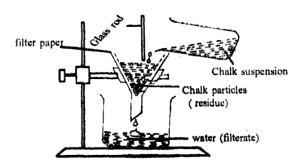
Filtration
It is used to separate soluble particles from liquids. It is used in water purification plants and breweries.
Evaporation
It is used to recover a solid solute from a solution
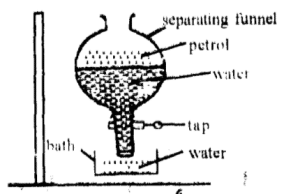
Separation Funnel
It is used to separate two immiscible liquid
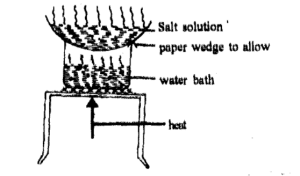
Crystallization
It is used to separate salts, which decompose on heating, from their solutions. The salt crystals obtained in this way are pure and usually contain water of crystallization.
It is applied in Industries where the purity of the product is important as in the manufacture of drugs and in sugar production.
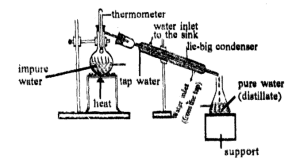
Distillation
It is used to recover a solvent from a solution. The solution is heated In a flask to vaporize the solvent.
Chromatography
It Is used to separate mainly coloured substances, Hero, a solvent move. Over porous adsorbent medium to separate a mixture of solutes.
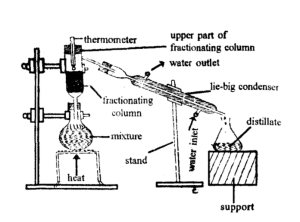
Fractional distillation
It is a form of distillation that is used to separate solvents based on their boiling points from their solution It uses fractionating tower (column) Note that the difference in boiling point between successive fractions must be more than 100°C.