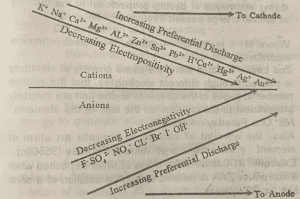
1. Positive of the ions in the electrochemical series
During electrolysis, those metals lower in the series are usually discharged in preference to those at the top.
-For non-metals, those higher up the series are discharged in preference to those below.
2. Concentration of Ions
When the concentration of an ion is increased, it promotes its chances of being discharged in preference to others. It can only be possible if the distance of the two competing ions is close in the electrochemical series.
NB: No matter the concentration of NaCL(aq) aqueous solution, the Na+ cannot be discharged in preference to H+ since the distance is not close in the electrochemical series.
3. Nature of electrode
The nature of electrode used in electrolysis can determine which ions to be discharged in preference to the others. For instance, when a mercuric electrode is used in the electrolysis of NaCL(aq), Na+ is discharged in preference to H+ since Na+ affinity to mercury is used as electrode.