These are devices that use stored chemical energy to generate electrical energy.
Electrode Potential
Standard electrode potential of a metal is the potential difference set up between metal and solution of its ions at 25°c (room temperature).
Electrode potential depends on:
(1) The temperature
(2) The overall energy change
(3) The concentration of ions in the solution
Types of electrochemical cells
Primary cells
These are cells that cannot be recharged e.g. simple cells, Le chlanche cell, Daniel cell etc.
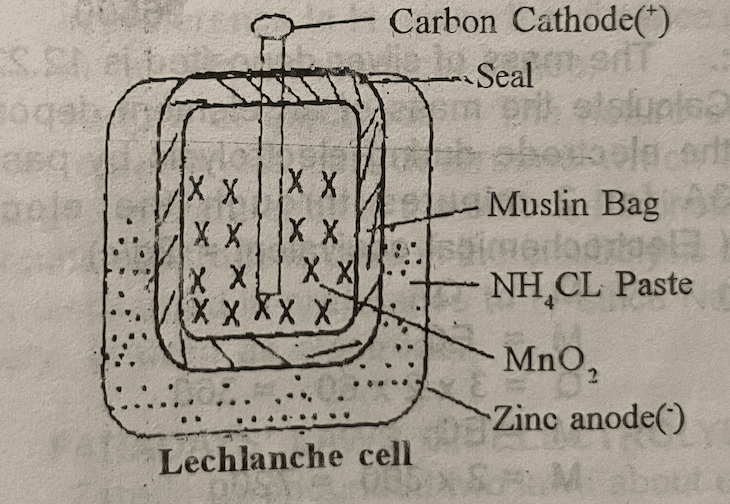
Some defects here are:
(1) Local action – It is due to impure zinc. It is corrected by the amalgamation of zinc before using it.
(2) Polarization – It is the accumulation of hydrogen bubbles at the Cathods which reduces the potential there. It is corrected by the use of depolarizers like MnO2.
Secondary cells
These are cells that can be recharged.
For instance, the lead acid accumulator, when recharged, they can generate a large current for a longer time.