The latest periodic table was the arrangement of elements in the increasing order of their relative atomic number. Modern periodic law states that the property of an element is the periodic function of its atomic number.
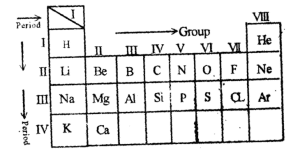
Group I — Are the alkali metals except for hydrogen.
Group II — Alkali earth metals.
Group VP — They are halogens or salt farmers.
Group VIII — They are noble gases or rare gases.
N.B: Transition elements are found between elements in groups two and three.
CHARACTERISTICS OF TRANSITION ELEMENTS
- They have variable valencies.
- They have a high melting point.
- They are paramagnetic.
- They change colour in solution.