The arrangement of electrons in an atom is governed by two rules:
(1) Hund’s rule — It states that electrons filling an orbital will first exist singly before pairing.
(2) Pauli exclusion principle — It states that not more than two electrons will occupy an orbital and these two electrons must spin in opposite directions.
Example:
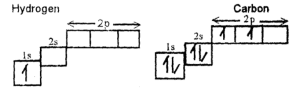
Modern Eletronic Configuration
| Sub shells | No. of orbital | Maximum electrons |
| S | 1 | 2 |
| P | 3 | 6 |
| D | 5 | 10 |
| F | 7 | 14 |
| ELEMENTS | CONFIGURATION | NO. OF ELECTRONS |
| Hydrogen | 1S1 | 1 |
| Helium | 1S2 | 2 |
| Lithium | 1S2, 2S1 | 3 |
| Beryllium | 1S2, 2S2 | 4 |
| Boron | 1S2, 2S2, 2P1 | 5 |
note: d block elements are transition elements. 4 F are lanthanides while 5 F are actinide elements.
Atomic number is the number of Proton in the nucleus of an atom. Z represents it.
Mass number is the sum of Proton and neutron present in the nucleus of an atom. A represents it.