AMMONIA: It is anhydrides of nitrogen. In nature, it is produced by decaying of nitrogenous waste in absence of air.
Laboratory Preparation of Ammonia
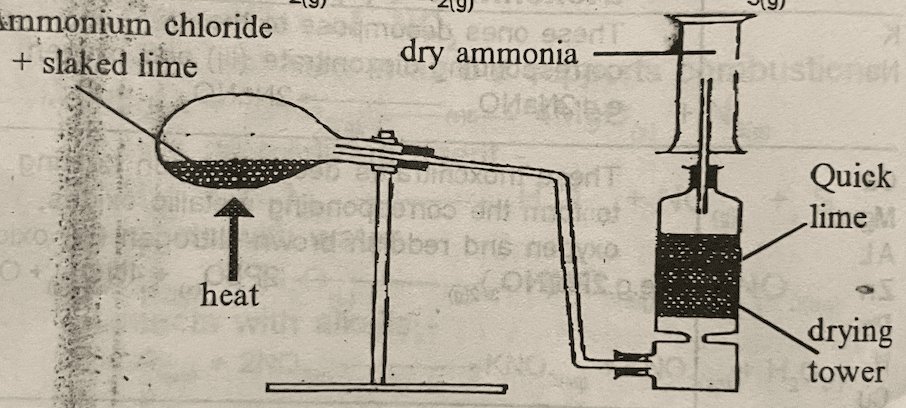
It is prepared in the laboratory by the action of slaked lime [Ca (OH)2] on ammonium chloride (NH4CL).
Ca(OH) + 2NH4CL(s) → CaCL2(s) + 2NH3 + 2H2O(L)
NB: Ammonia cannot be dried by drying like H2SO4(aq) and CaCL2(s) since they will react with it.
Industrially, ammonia is prepared by the combination of hydrogen and nitrogen in the ratio of 3:1 respectively by a process termed Haber process. It is performed at a temperature of about 450oc under the presence of about 200 atmospheres using finely divided iron as a catalyst. N2(g) + 3H2(g) → 2NH3(g) + heat.