Oxygen was discovered independently by Scheele in 1772 and priestly in 1774. It occurs abundantly on earth. It exists in both free and combined states.
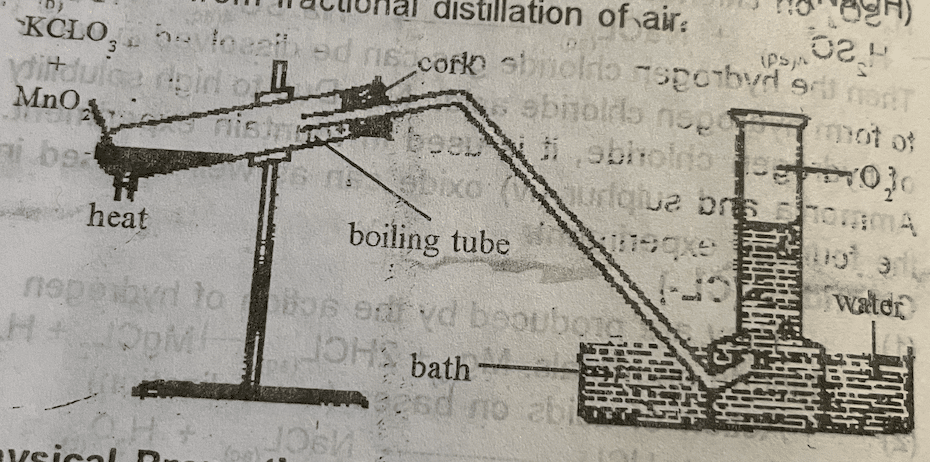
Laboratory Preparation of Oxygen
Oxygen is prepared laboratorily by the decomposition of potassium trioxochlorate (v) in the presence of manganese (iv) as a catalyst.
2KCLO3(s) Mno → 2KCL(aq) + 3O2(s)
Hydrogen to produce oxygen. Industrially, oxygen is obtained from the electrolysis of sodium hydroxide (NaOH) and also from the fractional distillation of air.