It is prepared by the action of a mineral acid on metals (those higher than hydrogen in the electrochemical series).
e.g.
Zn(s) + 2HCL(aq) → ZnCL2(aq) + H2(s)
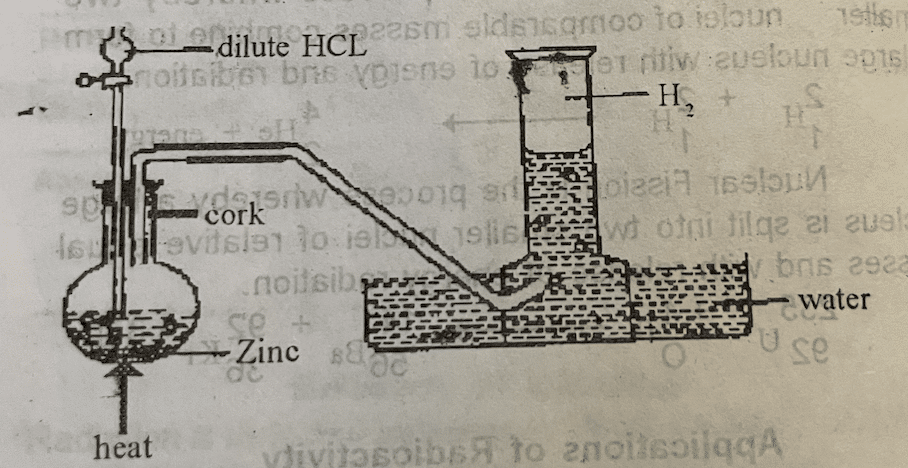
It is prepared by the action of a mineral acid on metals (those higher than hydrogen in the electrochemical series).
e.g.
Zn(s) + 2HCL(aq) → ZnCL2(aq) + H2(s)
