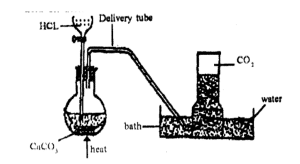
it is prepared in the laboratory by the action of mineral acid on trioxocarbonates.
CaCO3(S) + 2HCL(ag) →. CaCL2(ag) + H2O(L) + CO2(g)
Carbon (iv) oxide can also be obtained by heating of metallic tricocarbonates (except those of potassium and sodium),
Industrially, carbon (iv) oxide is obtained as a by-product in fermentation processes and when limestone is heated to make quick lime