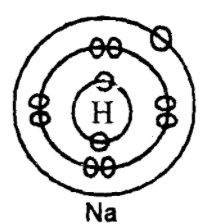
Electrovalent- It is a form of bonding whereby one or more electrons are transferred from one atom (metallic) to another atom (non-metallic) so as to attain either duplet or octet stable state. It is also called ionic bonding. It is found in Nacl Kcl, CaCL , CaO etc.
Example of Electrovalent Bonding
| BEFORE COMBINATION | |
 |
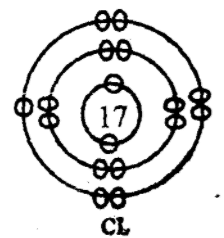 |
| Na | Cl |
| p = 11 | p = 17 |
| e = 11 | e = 17 |
| AFTER COMBINATION | |
 |
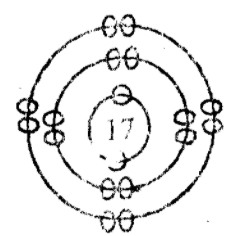 |
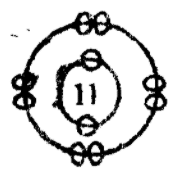
| Na | Cl |
| p = 11 | p = 17 |
| e = 10 | e = 18 |
Formation of sodium chloride, NaCl